11+ C2 Lewis Structure. Most lewis structures you encounter will be covalent bonds. Since valence electrons are typically represented as dots, these structural formulas sometimes are called lewis dot stutctures.
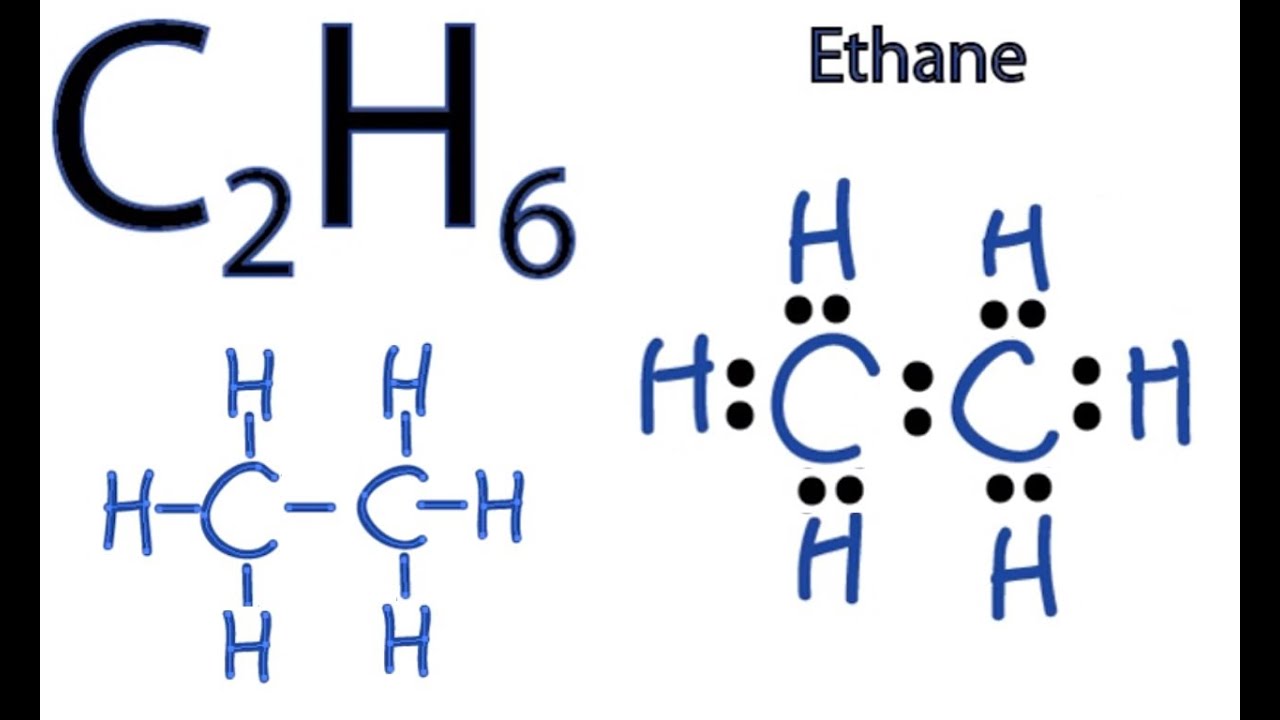
Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Writing lewis structures with the octet rule.
Once we know how many valence electrons.
11+ C2 Lewis Structure. What is the correct equation for showing the. 10.how many sigma and pi bonds are present in hcccho? B)two lone pairs of electrons. The lewis structure of c2, the chemical formula for diatomic carbon, is written with two cs connected by two straight lines.