11+ Ccl4 Lewis Structure. The lewis structure for ccl4 is a commonly tested lewis. To figure out lewis structure, do this:
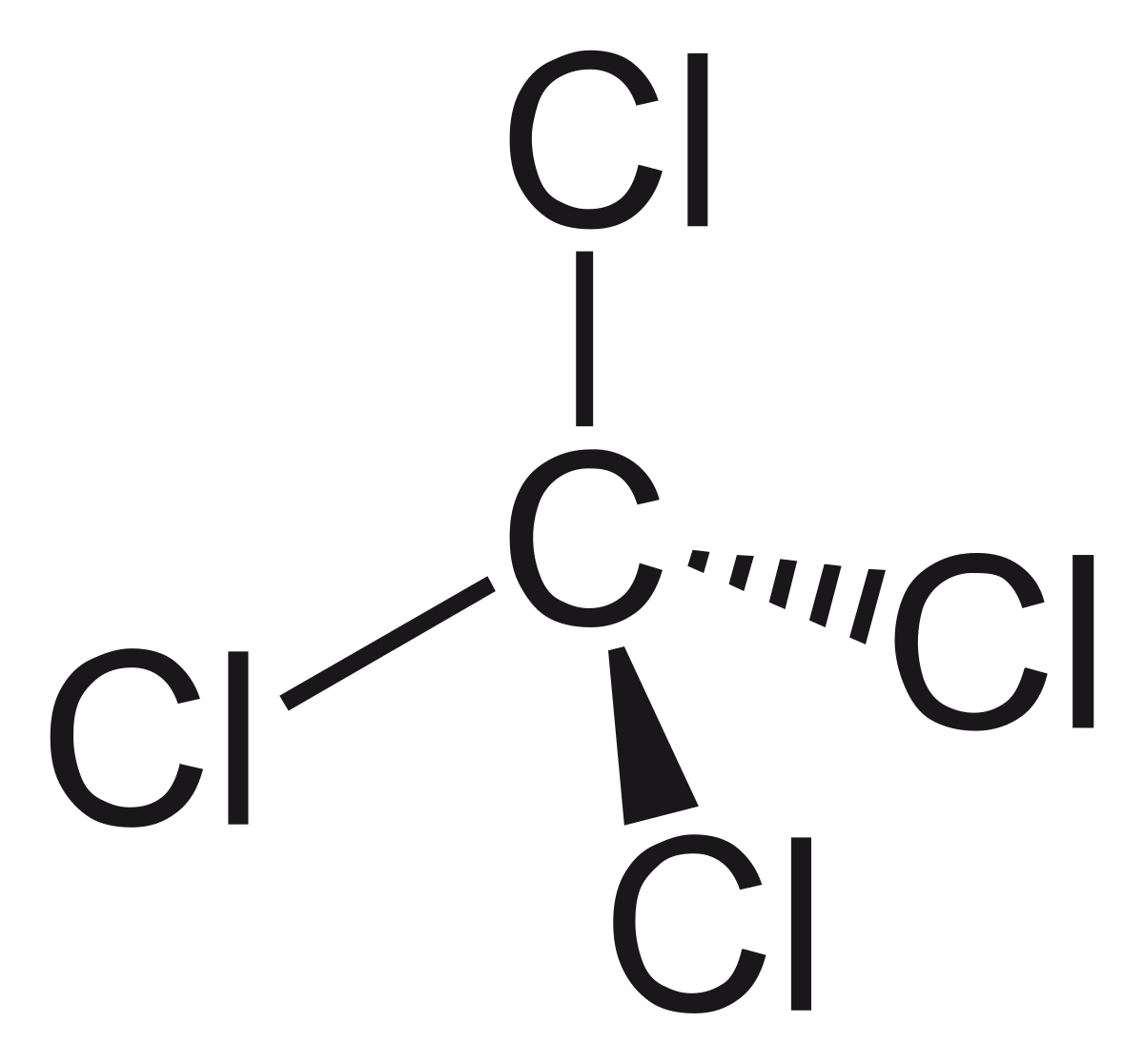
We could write it as well as a structural formula that would look like this right here. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl4 (carbon tetrachloride) and silicon in sih4 (silane). Lewis structures show how the electrons are arranged in the molecule.
The lewis structure for ccl4 is a commonly tested lewis.
11+ Ccl4 Lewis Structure. A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Draw an appropriate lewis structure for ch3sh. Four lines in the structure represent four bonds while dots around the chlorine atom represent valence electrons. 18 electrons trigonal planar bent.